
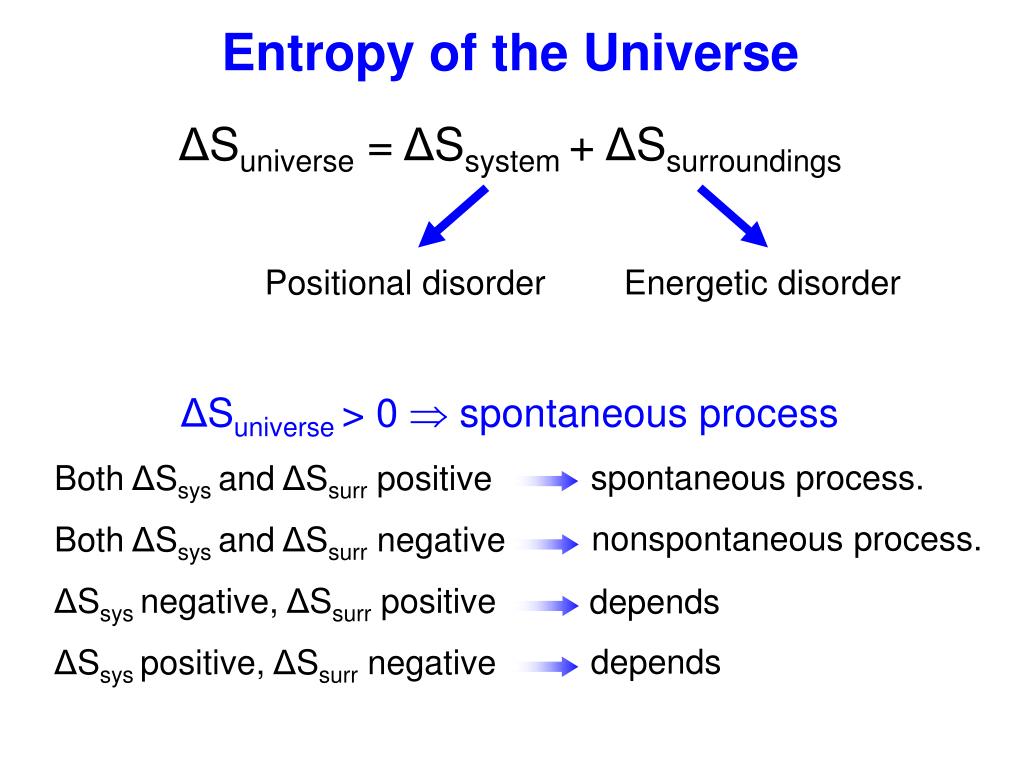
Entropy can have a positive or negative value. It is denoted by the letter S and has units of joules per kelvin. The value of entropy depends on the mass of a system. It measures the amount that has reacted ( $\xi$ = 1 mol means one mole has reacted if the coefficient is one, two when the coefficient is two and so on, with reactants having negative values and products positive values). Entropy is a measure of the randomness or disorder of a system. It is the extent of reaction $\xi$ (greek lowercase xi).

In your formal notation of dS, what is the physical quantity you took partial differential with?
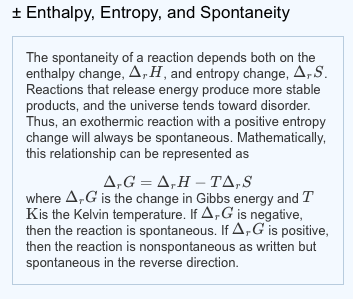
For example, in ligand substitution, an associative pathway is marked by low enthalpy of activation but a negative entropy of activation. These two parameters can be useful in understanding events leading to the transition state. For the nomenclature and symbols for Gibbs energy, see the first slide in this lecture. The activation entropy deals with how the energy within the molecule must be redistributed for the reaction to occur. Entropy increases as you go from solid to liquid to gas, and you can predict whether entropy change is positive or negative by looking at the phases of the. Textbooks, especially introductory once, are inconsistent in their nomenclature (there is an entire article on this topic for Gibbs energy of reaction). The activation entropy deals with how the energy within the molecule must be redistributed for the reaction to occur. when some reactant turns into some product according to the balanced chemical equation. The latter is a more formal way of writing your dS, signifying the infinitesimal small change in entropy as the reaction moves forward an infinitesimal amount, i.e. The temperature is constant.Īccording to $\Delta G = RT\ln(Q/K)$, $\Delta G$ will be highly negative at my initial conditions and will increase (become less negative) as reaction proceeds forward.Īlso, using the same expression, $$\Delta S = \frac$$ The former is what you can calculate from tabulated data. Now, suppose I start with 1 mole each of gases A and B, and zero (negligible) moles of C. Entropy is a measure of the randomness or disorder of a system. entropy, the measure of a system’s thermal energy per unit temperature that is unavailable for doing useful work.


 0 kommentar(er)
0 kommentar(er)
